Nitrogen is Continuously Moving Back and Forth Between the
1. Introduction: Nitrogen is important, but can be difficult to acquire
When you compare the nitrogen cycle to the carbon cycle, you encounter a paradox. Nitrogen makes up 78% of the atmosphere. When you feel the wind, 78% of what you're feeling is nitrogen gas, or N2. Carbon dioxide, by contrast, makes up less than one tenth of 1% of the atmosphere. But nitrogen is often the limiting nutrient in an ecosystem. In other words, the lack of nitrogen is what keeps the populations in those ecosystems from expanding their numbers.
The solution to the paradox boils down to this: despite nitrogen's abundance, it's difficult for living things to pull nitrogen from its atmospheric form as N2 into forms that living things can absorb. We'll see how nitrogen gets pulled into living things in a moment, but first let's review nitrogen's importance in living things.
Here's ATP. As I've noted elsewhere, ATP is possibly the most important molecule in living things, playing a key role in energy and information transfer. Note the nitrogen atoms in the nitrogenous base, adenine.
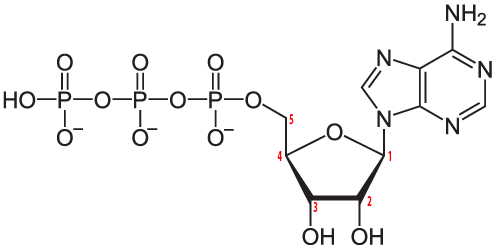
ATP is a nucleotide monomer. Nucleotide polymers like RNA and DNA also have nitrogenous bases as a key part of their structure.
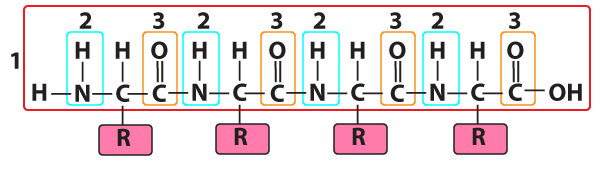
Here's the polypeptide backbone of a protein. Again, note the nitrogen. Nitrogen atoms also show up frequently in amino acid R groups (or side chains), with 6 of the 20 amino acid side chains things having one or more nitrogen atoms.
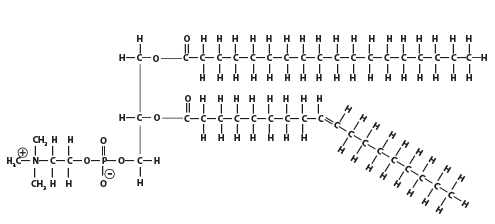
And here's a phospholipid: note the nitrogen atom in the polar head of the molecule.
So nitrogen is essential, abundant, and often scarce. Let's see why.
2. The Nitrogen Cycle: The Details
Because it's a cycle, we can start our study of the nitrogen cycle anywhere. But let's start with the atmosphere. Find the N2 at the top of the image below. Note that you can scroll the text below this image so that you can look at it as you read.
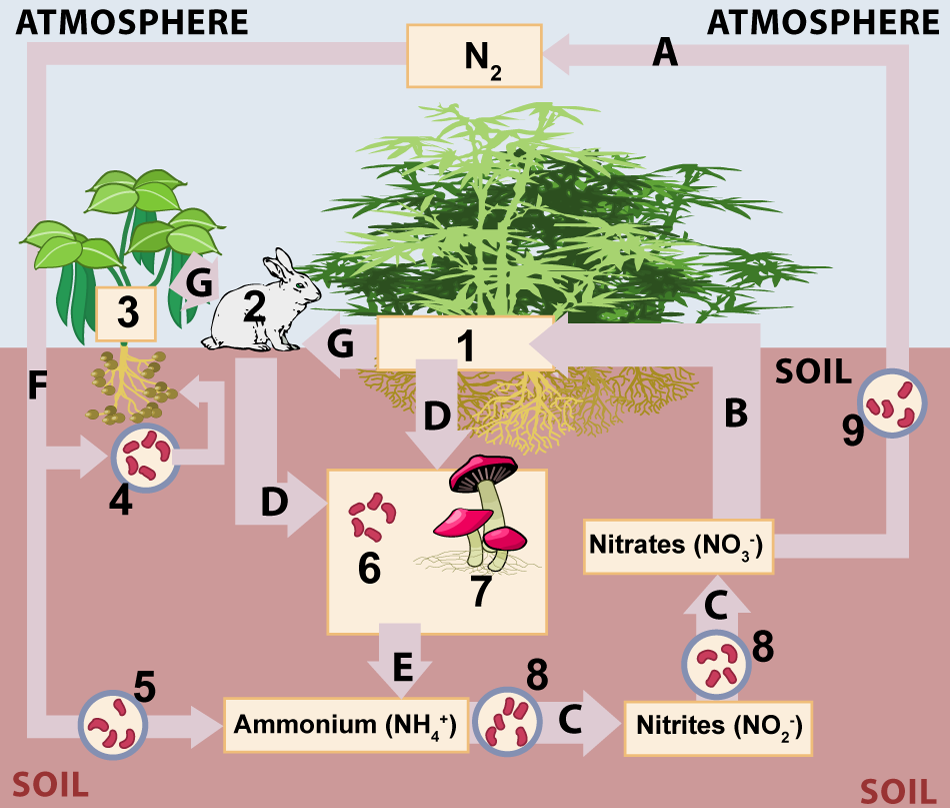
a. Nitrogen Fixation
In the atmosphere, molecular nitrogen (N2) is inert and unreactive. That's because the two nitrogen atoms together are held together by a triple bond: 3 shared pairs of electrons.
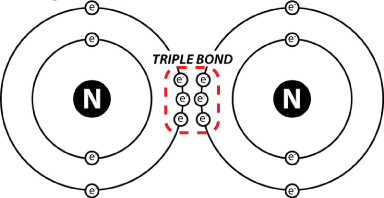
That is a very tight bond, and the only organisms that have evolved the ability to pry those two nitrogen atoms apart and attach them to something else are members of the domains bacteria and archaea. Bringing nitrogen into the biological realm is called nitrogen fixation, and the organisms that can perform this metabolic feat do so by using enzymes called nitrogenases.
Nitrogen fixation is shown at letter "F" in the diagram above. Take a look at the reaction that nitrogenases carry out, and notice two things about it (keeping in mind that this equation no more reflects the complexity of nitrogen fixation than "carbon dioxide + water –> glucose + oxygen" reflects the complexity of photosynthesis).

First, note that nitrogen fixation is endergonic. It takes a lot of ATP to drive this reaction forward.
Second, it's a reduction. Remember that oxidation is loss, and reduction is gain. Look at nitrogen, and note how on the left side it's all by itself, while on the right side it's attached to 3 hydrogen atoms, making it NH3. Note, also, the investment of hydrogen ions and electrons (a sure sign of reduction).
While the reaction above shows nitrogen fixation resulting in ammonia (NH3), it can also result in the ammonium ion (NH4 +).
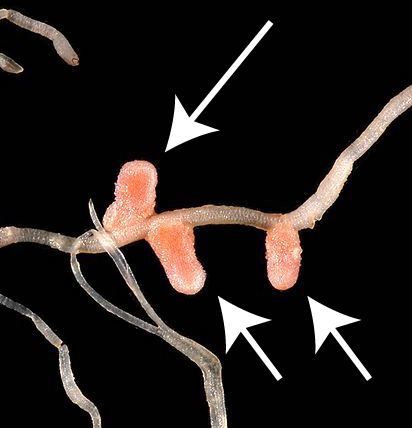
There are two microbial habitats where nitrogen fixing bacteria and archaea are found. One habitat is represented by the bacteria shown at number "4." These bacteria live in root nodules (swellings) in the roots of a family of plants known as legumes (represented by "3."). Root nodules are shown to your right. Legumes include plants like peas, beans, peanuts, and many other non-domesticated herbs, bushes, and trees.
The nodules are the site of a mutualistic exchange between the nitrogen-fixing bacteria that live in them and the plants that harbor them. Mutualism is a win-win relationship where both partners benefit. The bacteria benefit by receiving carbohydrates for energy and growth from the plants. The plants benefit by receiving nitrogen from the bacteria. In fact, plants with these symbiotic relationships with nitrogen-fixing bacteria are called nitrogen fixing plants (even though it's the bacteria that they harbor that do the actual nitrogen fixation).
A second microbial habitat where nitrogen fixing bacteria and archaea are found is the soil. These are shown at number "5."
Nitrification, Assimilation, and Consumption
Ammonium or ammonia in the soil is a source of energy, and this energy can be harvested by a variety of bacteria through a process called nitrification (C). Nitrification results in two oxidized forms of nitrogen: nitrates (NO3 –) and nitrites (NO2 –). Both of these forms of nitrogen can be readily absorbed by non-nitrogen-fixing plants (1) through a process called assimilation (B). Nitrogen can pass from these plants to animals by consumption (G). The two "G" arrows reflect the fact that animals can also eat nitrogen-fixing plants (something that you do every time you eat peas, soybeans, or peanuts).
Denitrification
Under anaerobic conditions, certain species of bacteria (indicated by "9") can reduce nitrates and nitrites back to nitrogen gas. This is called denitrification, and you can see it below as process "A." The result is a return of nitrogen gas to the atmosphere, completing the cycle.
Death and Ammonification
When organisms die (D), their nitrogen-containing molecules pass to bacterial and fungal decomposers (6 and 7, respectively). Decomposers render proteins and nucleic acids into the ammonium ion in a process called ammonification. That ammonia, in turn, can be nitrified into nitrates, and reassimilated into plants.
3. Nitrogen Cycle: Checking Understanding
4. The Nitrogen Cycle in Practice
In 1910, German chemists Fritz Haber and Carl Bosch invented an industrial process to pull nitrogen gas from the atmosphere and reduce it to ammonia (NH3). This process revolutionized agriculture by making it possible to cheaply produce nitrogen fertilizers. This removed a key limit on agricultural productivity, and is a major factor behind humanity's surging population growth in the last 100 years. Astonishingly, it's estimated that about 3.5 billion people are fed by food that humanity is able to grow because of this input of nitrogen into farms (source; https://ourworldindata.org/how-many-people-does-synthetic-fertilizer-feed)
In the quiz below, nitrogen fertilizer is added to the processes that you learned about above. Give it a try.
5. What's Next
- Continue to the next tutorial, Food Chains and Food Webs
- Click to return to the Ecosystems main menu. Or chose another module by using the College or AP Biology menus above.
schoenheimershichal75.blogspot.com
Source: https://learn-biology.com/ap-biology/ecology-tutorials/nitrogen-cycle/